The atom at the center and the eight partial atoms at the corners gives a total of two atoms in the unit cell of a body-centered cubic metal.
Click the link on the right to toggle between the contents of a unit cell and the lattice.
 |
BCC Metals | |
|
|
The Number of Atoms in the Cell 2 | |
An atom located on the lattice point in the center of this unit cell is completely within the cell. An atom on a lattice point at a corner of the cell is shared by a total of eight unit cells. Thus there would be one-eighth of an atom at each corner in this cell. The atom at the center and the eight partial atoms at the corners gives a total of two atoms in the unit cell of a body-centered cubic metal. Click the link on the right to toggle between the contents of a unit cell and the lattice. |
 One
atom is completely inside the unit cell.
One-eighth
of an atom is located inside the unit cell at each corner
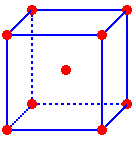 1 atom inside the cell + 8 corners * (1/8) atom at each corner = 2 atoms per cell
|
|
Copyright ©
2003 by: Journal of Chemical Education Software |
||