
An atom at the corner of this unit cell would be shared by a total of eight unit cells. Thus there would be one-eighth of an atom at each corner in this cell. The other seven-eighths of each atom are located in the adjacent unit cells
One simple cubic unit contains the equivalent of one atom.
Click the links on the right to illustrate the concepts above.

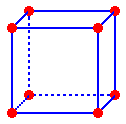
8
corners per cell * 1/8 an atom in each corner = 1 atom per cell
One
eighth of an atom is located inside the unit cell at each corner.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software