
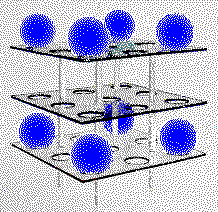
This model of a simple cubic unit cell is constructed of Plexiglas plates with holes to hold spheres that represent atoms. The spheres are located at the corners of a cube in this model.
While such a model is helpful in looking at the geometry of a metal, it is important to remember that many features of such models are not exactly correct. Atoms are not hard spheres. The spheres (atoms) in this model do not touch, but the atoms touch along the edges of the unit cell in a simple cubic metal and there is only one-eighth of an atom inside the cell at each corner.
Click the link on the right to highlight an edge.

Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software