
Click the links on the right for a short review. They are numbered for your convenience.
a,
the length of
the unit cell edge
the unit cell edge
R,
the radius of the
atom
atom
R=
1/2 a
a =
2 R
2 R
A
portion of the structure of a simple cubic metal

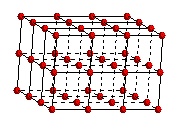
A
simple cubic space lattice
Atoms
are located at the lattice points in a simple cubic metal
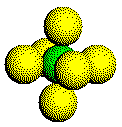
Atoms
in a simple cubic metal have a coordination number of six

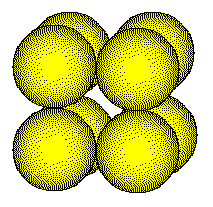
One
eighth of an atom is located inside the unit cell at each corner giving
a total of one atom per unit cell
Atoms
are located at the lattice points in a simple cubic metal
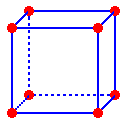
A
simple cubic unit cell is a cube with lattice points at the corners
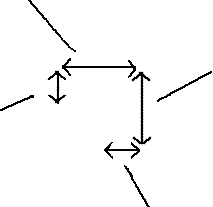
The
edge length of a simple cubic unit cell is twice the radius of the atoms
in the cell
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software