
Most drawings of unit cells use small spheres to represent atoms so that the atoms inside the cell are visible. Thin lines are sometimes used to help indicate the position of the ions in the cell.
Click the links on the right to illustrate the above concepts.
Thin lines are sometimes used to help locate the ions
in the cell.
The
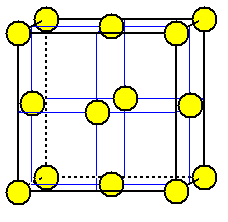
unit cell of ZnS is a drawn cube
Sulfide ions are placed on the eight corners
of the unit cell and in the center of each face.
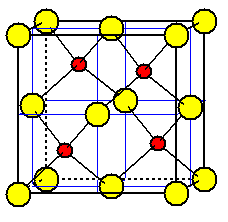
Sulfide ions are placed on the eight corners
of the unit cell and in the center of each face.
Zinc ions are placed in one half of the tetrahedral holes formed by sulfide ions.
Copyright ©
2003
by: Journal of Chemical Education Software
by: Journal of Chemical Education Software