|
|
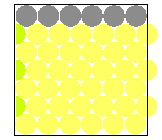 |
Square vs close packing
More atoms fit in the same area when they are close packed rather than square packed. This movie shows the shifting of atoms from the less efficient square packing to the more efficient close packing. This demonstration can be performed using, for example, a 6 x 6 grid of pennies on an overhead projector.
|
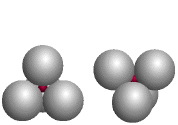 |
Tetrahedron
The red atoms fill tetrahedral holes formed by four identical larger atoms; the coordination number of each red atom is four. (Companion Figure 5.18)
|
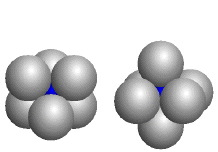 |
Octahedron
The blue atoms fill octahedral holes formed by six identical larger atoms; the coordination number of each blue atom is six. (Companion Figure 5.14)
|
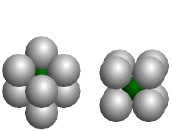 |
Cube
The green atoms fill cubic holes formed by eight identical larger atoms; the coordination number of each green atom is eight. (Companion Figure 5.11)
|
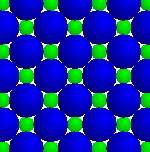 |
Radius ratio
A range of radius ratios will prevent like-charged ions from touching and making the lattice unstable; in this case, 2.414 > r2/r1 > .414 (octahedral coordination).
|
 |
Model spheres
The 4 sizes of spheres in the ICE Solid State Model kit, whose radii reflect ideal packing ratios in many mineral structures. If the clear spheres at the left (1 inch diameter) are packed in the shape of a cube, the green sphere (0.732 inch diameter) fits snugly in the cube's center; if the clear spheres are packed in the shape of an octahedron, the blue sphere (0.414 inch diameter) fits snugly in the octahedron's center; and if the clear spheres are packed in the shape of a tetrahedron, the pink sphere (0.225 inch diameter) fits snugly in the tetrahedron's center. (Companion Figure 5.10)
|
 |
Tetrahedral and octahedral models
A view of the tetrahedral hole (left) and a view of the octahedral hole (right), constructed with the ICE Solid State Model Kit. (Companion, Figure 5.18A and 5.14A)
|
 |
Octahedral model
A view of the octahedral hole, constructed with the ICE Solid State Model Kit. (Companion, Figure 5.14B)
|
 |
Cubic model
A view of the cubic hole, constructed with the ICE Solid State Model Kit. (Companion, Figure 5.11)
|
 |
fcc unit cell
Copper has the CCP structure and many close-packed planes that facilitate plastic deformation (slip planes). (Companion Figure 5.9) A portion of a structure that when repeated by moving it parallel to its edges by the length of an edge generates the entire crystal structure (without overlapping) is called a unit cell. When atoms are located on the corners, edges, or faces of the unit cell, only fractions of those atoms belong in the unit cell. The "unit cell" shown in this animation is actually the smallest collection of spheres that contains a single unit cell.
|
 |
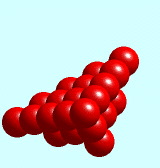
fcc slip planes
Close packed slip planes in an fcc unit cell are revealed by lifting any of the lower corners of the Solid State Model Kit fcc unit cell
|
 |
fcc to ccp
A face centered cubic structure is equivalent to a cubic close packed structure. The movie starts with an fcc view showing more than one unit cell for the NaCl structure (one unit cell is outlined in the first frame) and rotates to show a close packed layer.
|
 |
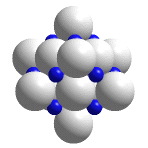
Salt cleavage
When an ionic structure is deformed so that like charges are adjacent to one another, the structure cleaves. The force of the blow is not nearly as important as the orientation. (Companion Experiment 2)
|
 |
NaCl body diagonal unit cell
The NaCl crystal tends to cleave along the faces of the cubic unit cell. A portion of a structure that when repeated by moving it parallel to its edges by the length of an edge generates the entire crystal structure (without overlapping) is called a unit cell. When atoms are located on the corners, edges, or faces of the unit cell, only fractions of those atoms belong in the unit cell. The "unit cell" shown in this animation is actually the smallest collection of spheres that contains a single unit cell
|
 |
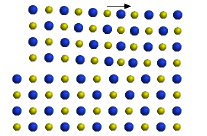
NaCl model
The ICE Solid-State Model Kit is based on radius-ratio-size spheres. Structures are built by sliding spheres down rods one layer at a time. Assembly of the rock salt structure is illustrated. (Companion Appendix 5.1)
|
 |
Salt cleavage
When an ionic structure is deformed so that like charges are adjacent to one another, the structure cleaves. (Companion Figure 6.1)
|
 |
Diamond pencil
A diamond scribe scratches a piece of glass. (Companion Demonstration 7.8 and Experiment 2)
|
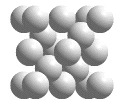 |
C(diamond) unit cell
In diamond, each carbon atom is covalently bonded to four other carbon atoms in a tetrahedral geometry. A portion of a structure that when repeated by moving it parallel to its edges by the length of an edge generates the entire crystal structure (without overlapping) is called a unit cell. When atoms are located on the corners, edges, or faces of the unit cell, only fractions of those atoms belong in the unit cell. The "unit cell" shown in this animation is actually the smallest collection of spheres that contains a single unit cell.
|
 |
Graphite Pencil
The carbon layers of graphite are held together by weak van der Waals forces, allowing them to slide easily relative to each other. (Companion Experiment 2)
|
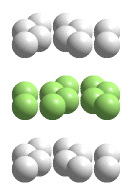 |
C(graphite) unit cell
Graphite's strong covalent bonds lie entirely within planes, forming a two-dimensional layered solid. The spacing between the atoms in the layers and between the layers is to scale. A portion of a structure that when repeated by moving it parallel to its edges by the length of an edge generates the entire crystal structure (without overlapping) is called a unit cell. When atoms are located on the corners, edges, or faces of the unit cell, only fractions of those atoms belong in the unit cell. The "unit cell" shown in this animation is actually the smallest collection of spheres that contains a single unit cell
|
 |
MoS2
A piece of cellophane tape can be used to peel off layers of molybdenum sulfide. (Companion Demonstration 5.5)
|
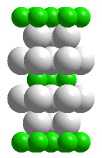 |
MoS2 unit cell
Layers of sulfur atoms that do not sandwich molybdenum atoms are in direct contact with each other. These like-charged sulfur layers repel each other, and are held together by weak van der Waals forces. A portion of a structure that when repeated by moving it parallel to its edges by the length of an edge generates the entire crystal structure (without overlapping) is called a unit cell. When atoms are located on the corners, edges, or faces of the unit cell, only fractions of those atoms belong in the unit cell. The "unit cell" shown in this animation is actually the smallest collection of spheres that contains a single unit cell.
|
 |
CaF2 (octahedral crystals)
When the cubic unit cell faces of calcium fluoride grow most rapidly, an octahedral habit can be produced. (Companion Appendix 5.5)
|
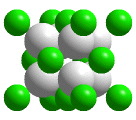 |
CaF2 unit cell
The cubic unit cell of calcium fluoride. (Companion Figure 5.20) A portion of a structure that when repeated by moving it parallel to its edges by the length of an edge generates the entire crystal structure (without overlapping) is called a unit cell. When atoms are located on the corners, edges, or faces of the unit cell, only fractions of those atoms belong in the unit cell. The "unit cell" shown in this animation is actually the smallest collection of spheres that contains a single unit cell
|
 |
PbS
The cubic habit of lead sulfide, which crystallizes in the NaCl (rock salt) structure
|
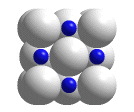 |
NaCl unit cell
The cubic unit cell of lead sulfide. A portion of a structure that when repeated by moving it parallel to its edges by the length of an edge generates the entire crystal structure (without overlapping) is called a unit cell. When atoms are located on the corners, edges, or faces of the unit cell, only fractions of those atoms belong in the unit cell. The "unit cell" shown in this animation is actually the smallest collection of spheres that contains a single unit cell.
|
 |
Si wafer
A silicon wafer is flexed until the wafer shatters. The directional covalent bonding of silicon leads to brittleness. Contrast this with bending copper (metallic bonding) and with cleaving NaCl (ionic bonding). (Companion Demonstration 6.1)
|
 |
C(diamond) unit cell
In silicon, which has the diamond structure, each silicon atom is covalently bonded to four other silicon atoms in a tetrahedral geometry. A portion of a structure that when repeated by moving it parallel to its edges by the length of an edge generates the entire crystal structure (without overlapping) is called a unit cell. When atoms are located on the corners, edges, or faces of the unit cell, only fractions of those atoms belong in the unit cell. The "unit cell" shown in this animation is actually the smallest collection of spheres that contains a single unit cell.
|
 |
Si wafer closeup
Close up of a silicon wafer flexed until the wafer shatters. The directional covalent bonding of silicon leads to brittleness. Contrast this with bending copper (metallic bonding) and with cleaving NaCl (ionic bonding). (Companion Demonstration 6.1)
|

