
In this post, I’m sharing the materials I created for an electrochemistry mini-unit that I used in my AP Chemistry interactive notebook. It’s important to note that you do not need to do interactive notebooking with your students to find some or all of these materials useful. I have included the following:
- a side-by-side comparison of all the features of galvanic and electrolytic cells
- Topic 9.7 - Galvanic (Voltaic) and Electrolytic Cells
- Learning Objective ENE-6.A
- a foldable that describes the purpose of each equation that applies to electrochemical cells
- Topics 9.8 - 9.9 - Cell Potential and Free Energy, Cell Potential Under Nonstandard Conditions
- Learning Objectives ENE-6.B and ENE-6.C
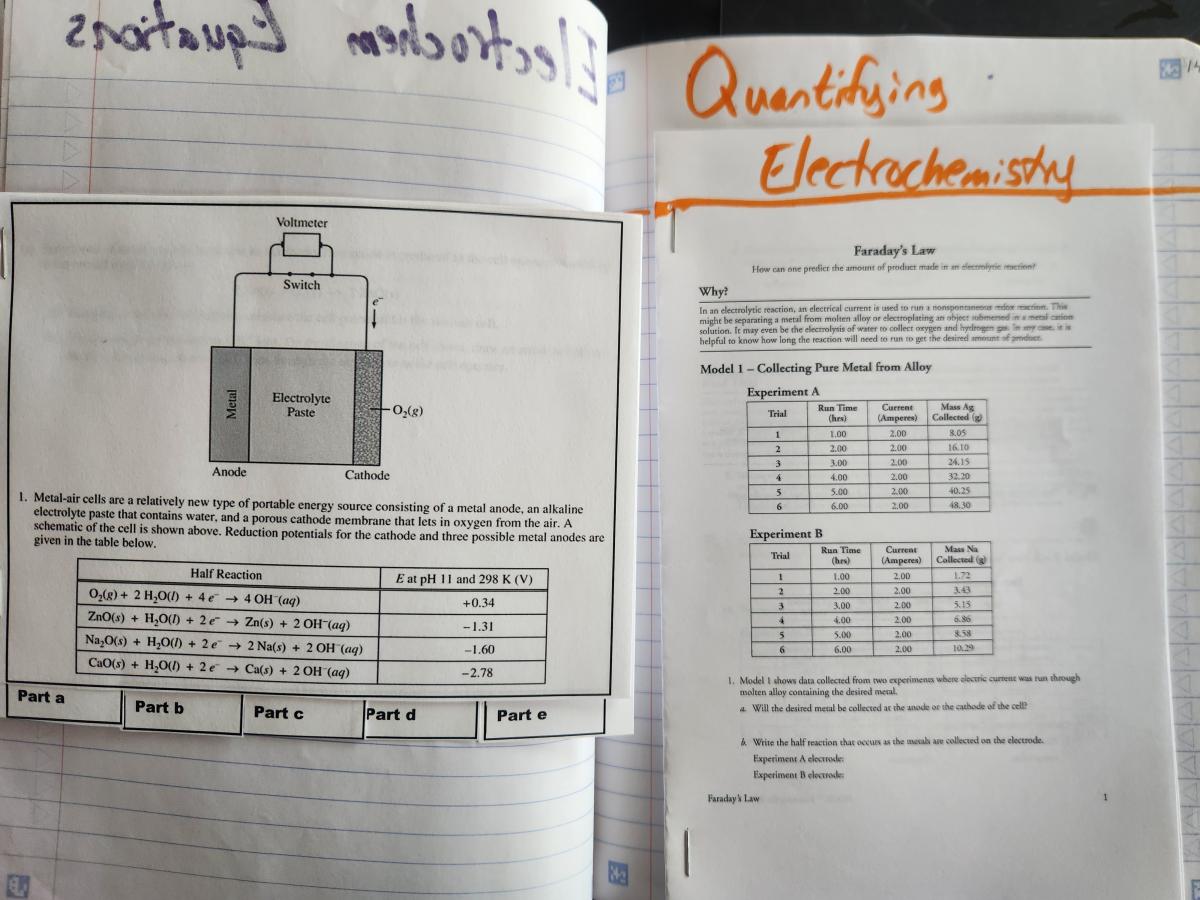
- Faraday’s Law POGIL (I used this POGIL, which can be found in the POGIL Activities for AP Chemistry book.)
- Topic 9.10 - Electrolysis and Faraday’s Law
- Learning Objective ENE-6.D
I’m continuing my ever evolving journey with interactive notebooks. This year, I committed to creating an interactive notebook in my AP Chemistry class. I focused on using it as a way to highlight relationships between various models that we use in AP Chemistry, including equations, graphs, particle representations, data tables, and diagrams. I also wanted to be intentional in creating a layout that would more concretely link AP Practice problems and the content to facilitate connections. To meet this goal, I print AP practice problems and use them on the left side of each spread, with the corresponding content page on the right. I use Topic Questions from AP Classroom, portions of AP free responses questions, and questions from publicly released practice tests. On some pages I had students review sample student responses from released free response questions and identify strengths and weaknesses of the responses. In other places I use lab activities. I do my best to make sure that students see a mix of both multiple choice and free responses questions throughout the unit.
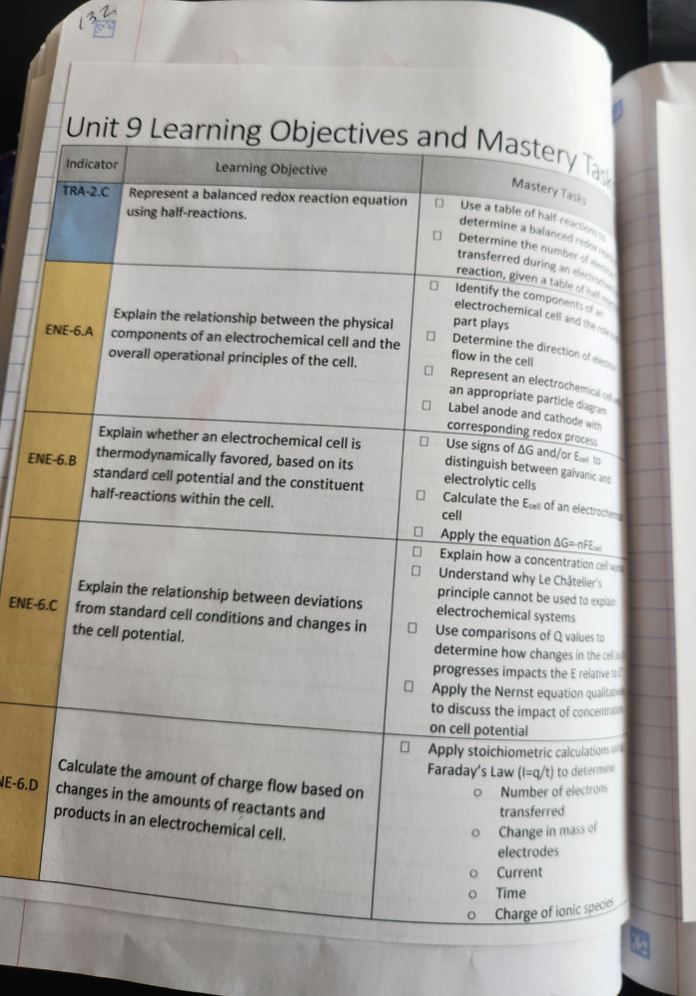
As I have discovered before when I create interactive notebook pages, this process helped me to see new ways to present the content. Additionally, thinking about how to unpack the AP learning objectives to translate them into notebook pages led me to create a list of student mastery tasks for each unit. I now print this for each unit and tell them to treat it as a checklist.
In the future, I will be refining and continuing to share my original AP notebooking materials. I hope that you and your students find them useful!
If you have questions or comments, please log into your ChemEd X account and add a comment below the post.
Composition notebooks, printed copies of each page, colored paper or construction paper for making flipbooks/foldables, scissors, tape or glue
**A note on my manipulatives: If you see a dashed line, that is where the item should be cut. If you see dotted lines, that means fold.
For backstory about how Nora uses interactive notebooks, view a recording of her ChemEd X Talk: Integrating Interactive Notebooks into Chemistry Courses with Nora Walsh and read her previous post, Interactive Notebook Unit on Stoichiometry. Nora wrote the following in response to questions she received after her ChemEd X Talk: A Classroom View of Using Interactive Notebook Pages.
See all of the interactive notebook units Nora has published: INTERACTIVE NOTEBOOK COLLECTION
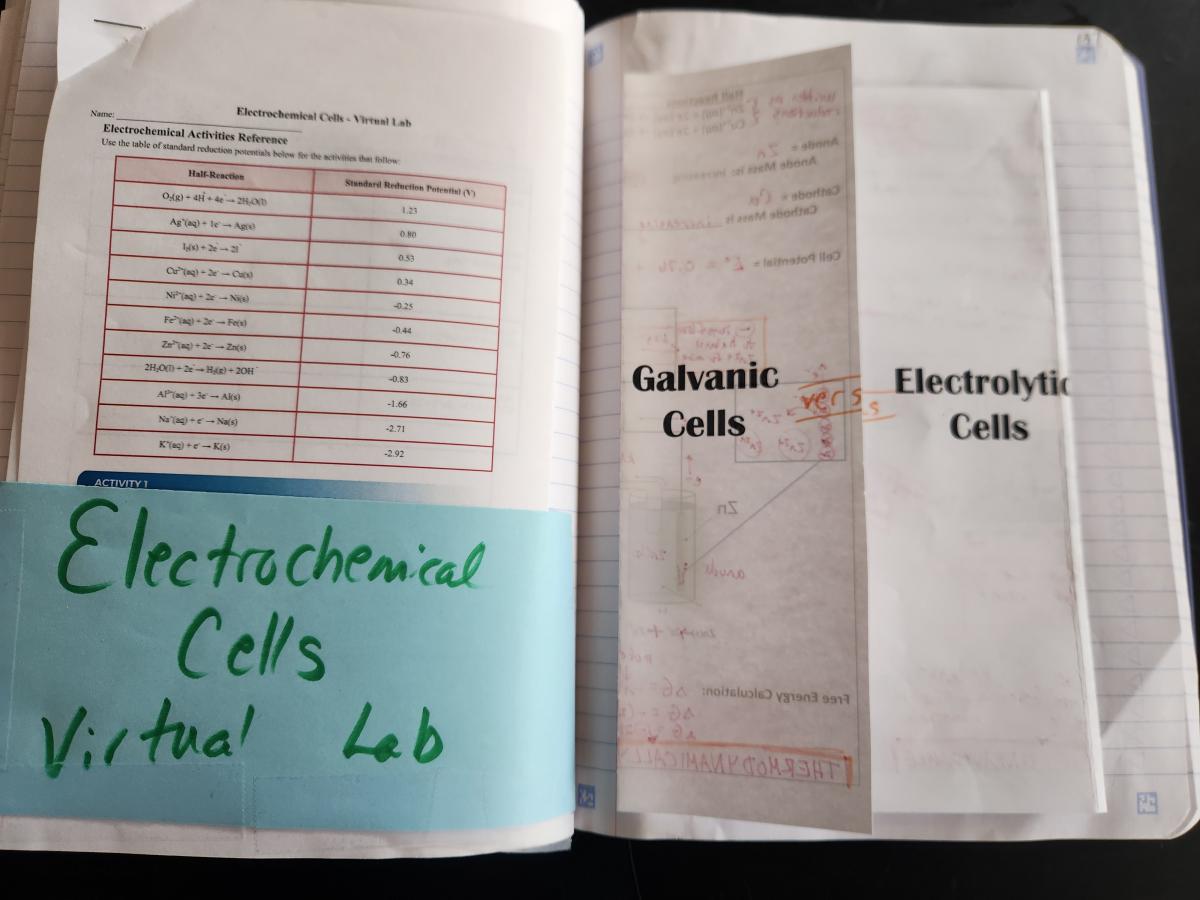
Spread 1: Unit Introduction
Leftside - Learning Objectives and Mastery Tasks
RIghtside - Title Page
Spread 2: Galvanic and Electrolytic Cells
Left side - Instead of using the AP questions on this layout, I used the Galvanic/Voltaic Cells Virtual Lab from AACT. I displayed the simulation as we went through the foldable on the right side so students could see the cells in action. There is an accompanying write-up on AACT for members that can be used, or you could use the simulation to go along with pretty much any Galvanic Cells Lab write-up.
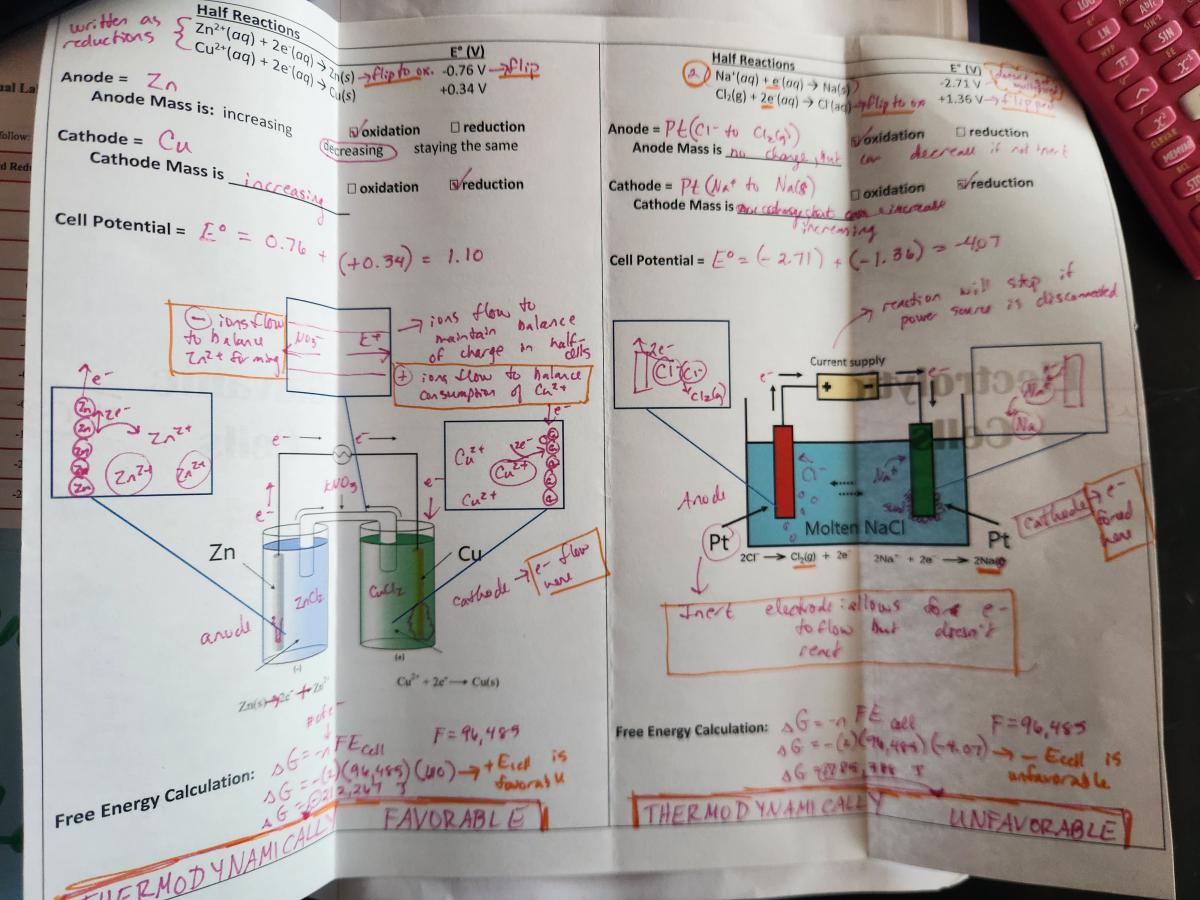
Right side - Galvanic versus Electrolytic Cells Shutter foldable - This is a direct side-by-side comparison of all of the content that is often linked with electrochemical cell diagrams on test questions. There is a LOT of information in this foldable, but it helps students to see the vast number of ways that cells can be described (again, relating several different models: net ionic equations, calculations, particle diagrams, data tables). It took my class two 45 minute class periods to go all the way through this foldable and the accompanying virtual lab.
Spread 3: Cells at Standard and Nonstandard Conditions
Left side - AP Topic Questions for Topics 9.8 and 9.9
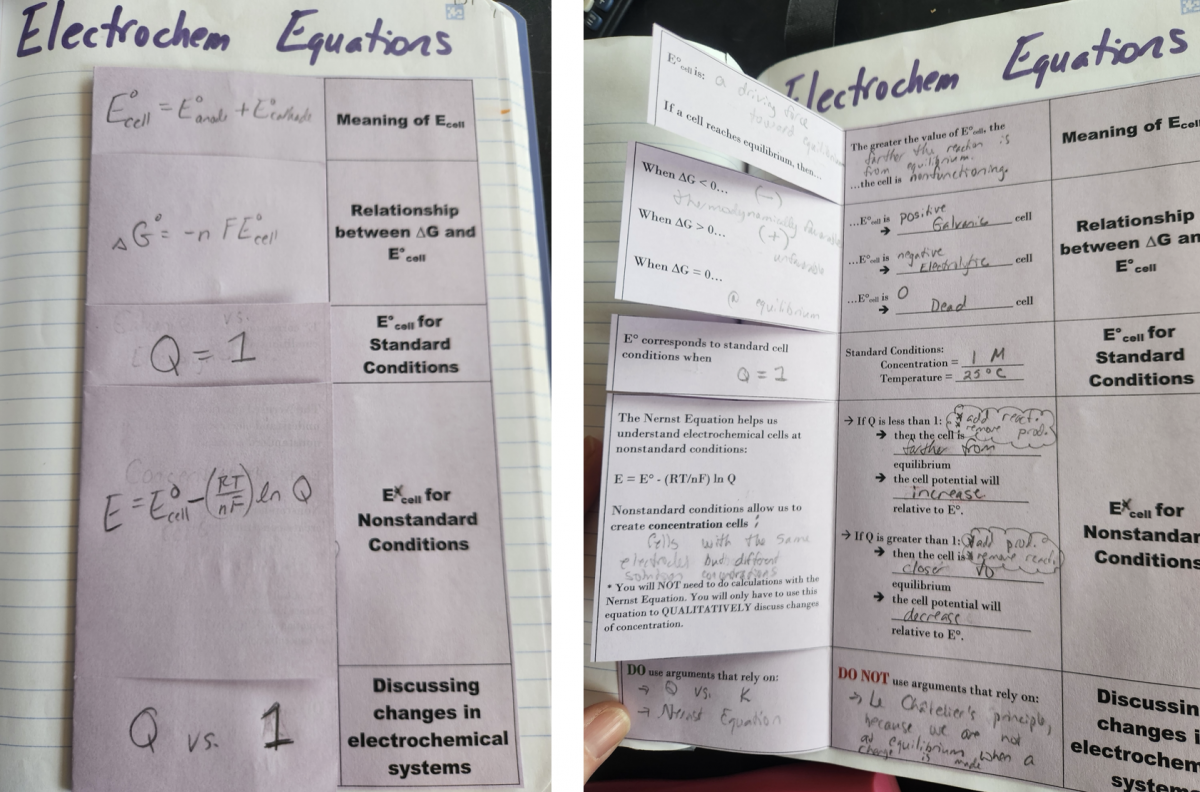
Right Side - This foldable relates all of the electrochemical equations except for electrolysis calculations. This may sound a bit repetitive, as some of these equations are applied in the Galvanic versus Electrolytic foldable, but I really wanted to drive home the way these equations can be related to each other. When I began creating this page to cover Topic 9.8-9.9, I wasn’t quite sure where I was going to land with the formatting. Once I started getting the notes onto paper I realized the model linking them all together was mathematical expressions. In the end, this page is a really powerful way to cement the meaning of the equations and how to leverage them for justifications.
Spread 4: Faraday’s Law
Left side - 2015 Operational Exam - FRQ #1 - This question is great to cover a lot of electrochemistry topics all in one, as well as a few things from unit 1. I’m a big, big believer in spiraling throughout the AP Chemistry course so that exam review is a refresh instead of a relearn. This question can be accessed either on AP Classroom or on the College Board website. I formatted it as a tab book to make it fit on a single page, which can be accessed below.
Right side - Faraday’s Law POGIL - I’m a fan of POGIL activities, and they work very, very well in an interactive notebook. This is a really great one for helping students to make stepwise connections between prior skills and new content for electrolysis.
See the video to help visualize what these pages will look like in action.
Provide copies of the following for each student. Note that I have grouped the components by page. Please preview them before printing as some pages have multiple copies to minimize paper waste.
![]() AP Electrochemistry INB RUBRIC WORD and AP Electrochemistry INB RUBRIC PDF
AP Electrochemistry INB RUBRIC WORD and AP Electrochemistry INB RUBRIC PDF
Spread 1: ![]() Unit 9 Electrochem Learning Objectives
Unit 9 Electrochem Learning Objectives
Spread 2: ![]() Galvanic vs. Electrolytic Cells
Galvanic vs. Electrolytic Cells
Spread 3: ![]() Cells at Standard and Nonstandard
Cells at Standard and Nonstandard
Spread 4: ![]() AP Problem- 2015 #1
AP Problem- 2015 #1
N/A

All comments must abide by the ChemEd X Comment Policy, are subject to review, and may be edited. Please allow one business day for your comment to be posted, if it is accepted.
Comments 1
Interactive notebook for electrochemistry
This is a fabulous idea that will increase student understanding and student learning. I endorse it wholeheartedly . . .