|
Dear Readers, This is my 21st year at my school district (following a few random years at other schools and a couple years in a chemistry lab). I have been reflecting on my career a lot lately. I came into the classroom feeling like I knew all there was to know about teaching and that once I had put in my dues creating lessons for a few years, the work would get easier. However, it took me about seven years to really put all the pieces (curriculum, content, lab prep, supply orders, professional development, discipline, technology...) together into a truly effective package. That said, the work has not gotten easier. By the end of that seventh year, I realized that continued education and professional development are crucial to maintaining my effectiveness. Years when my health and personal life, my students, my admin and colleagues are all positive, teaching is certainly easier. A glitch in one or more of those areas can negatively effect everything else, but it is during those times that a strong professional network can be most important. I encourage new teachers to work on building that network. For veteran teachers like myself, I hope you will invite some new blood into your fold so they can benefit from your experience and let those that you have relied on know how important they have been. Gratitude is a gift to the sender as much as the receiver. I am re-energized as I am finishing up a week long break from school and ready to get back to my students in the next few days, I am ready to spend the next eight weeks pulling everything together and putting the finishing touches on a good year. Happy Earth Day! Deanna Cullen
|
||
|
So You Think You Can Demo - Contest As teachers, we perform every day. How about performing a demonstration on video and submitting it to the "So You Think You Can Demo" contest!? |
||
|
|
||
|
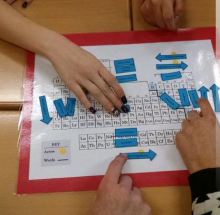
Dan Meyers shares a game that helps his students review the trends on the periodic table. Read the post for more details. Instructions and the game are available for download. |
||
|
|
||
|
How does an orange peel pop a balloon? Chemistry, of course! The juice from an orange peel causes a balloon to pop. When Tom Kuntzleman first saw this effect, he immediately thought to himself, “what is the chemistry involved in this experiment?” After quickly searching the web, he found several claims that a compound in orange peels called limonene (Figure 1) is responsible for this effect. Limonene is also responsible for the wonderful smell of oranges, and it is a liquid at room temperature. READ MORE... |
||
|
|
||
|
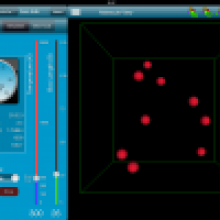
Using technology to help students understand Maxwell-Boltzmann Distribution Curves Lowell Thomson shares his experience using an iPad simulation and a Java applet to introduce his students to Maxwell-Boltzmann distribution curves. |
||
|
|
||
|
Michael Morgan has developed a passion for leading a Chemistry Olympiad team. Read about his experience and find out more about this program. |
||
|
|
||
|
Journal of Chemical Education April 2015 The April issue has just been released. Many of the articles were specifically chosen for this issue because they relate to the Chemists Celebrate Earth Day theme. Mary Saecker highlights the manuscripts published in this months edition and also offers related resources from the JCE archives. The cover photo is related to an article co-authored by our own Tom Kuntzleman. You might also be interested in his recent blog post about a dry ice demo. |
||
|
|
||
|
Planning My Professional Development ChemEd is specifically designed for high school level chemistry teachers. Consider attending this summer in Georgia! Registration is now open. Early bird registration rate is available until May 1. |
false | |
|
|
||
|
We hope you enjoy the content mentioned here and other content at ChemEd X. If you find ChemEd X content useful, please consider purchasing a subscription to help support ChemEd X. In addition to supporting the free content we make available, by subscribing you will also gain access to our complete video collection and other titles to help in teaching and learning chemistry.
|
||