
The Aufbau Principle: the (n + l) Rule
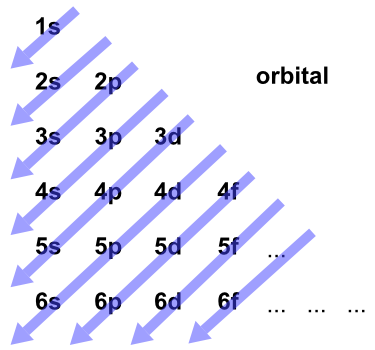
We’ve all seen and use the so-called Aufbau Diagram (Figure 1). It is a mnemonic used to remember the order of “filling” of atomic orbitals during the construction of the ground state electron configurations of the elements. The presentation of this diagram is largely disconnected from any physical meaning. Here’s what we tell our students: “Memorize the diagram, learn to use it, and you’re guaranteed to get the right answer.”
Figure 1. The Aufbau Diagram: Atomic orbitals are filled starting at 1s and continuing, from the upper left, in the order indicated by the arrows.
Is there a way to connect this diagram to its physical meaning? Yes! That is the goal of this article.
How was this diagram constructed in the first place? It turns out that it is a representation of a method of predicting the “order of filling” called the Madelung rule, which is also called the (n + l) rule. The “n” and “l” in the (n + l) rule are the quantum numbers used to specify the state of a given electron orbital in an atom. n is the principal quantum number and is related to the size of the orbital. l is the angular momentum quantum number and is related to the shape of the orbital.
Here’s how the (n + l) rule works. The (relative) energies of the orbitals can be predicted by the sum of n + l for each orbital, according to the following rules:
a. Orbitals are filled in order of increasing (n + l), which represents the relative energy.
b. If two orbitals have the same value of (n + l), they are filled in order of increasing n.
The diagram in Figure 1 is the result of these rules.
Figure 2 is a version of the diagram that displays the dependence on (n + l) for each orbital, where E represents the relative energy of the orbitals. The orbitals are filled according to the values of E for each orbital: E=1 for 1s, E=2 for 2s, E=3 for 2p and 3s, and so on. According to rule (b) above, when two orbitals have the same E, such as E=3 for 2p and 3s, the orbital with lower n (2p) is filled first.
Figure 2. An Aufbau diagram that illustrates the (n+l) rule.
The (n + l) rule is a remarkably clever and useful tool. It correctly predicts the order of orbital energies through element 20 (calcium). It also correctly predicts many electron configurations beyond that. And here we arrive at a very important point: predicting the relative energies of each orbital is not the same thing as predicting correct electron configurations. More on this point later.
Why does the (n + l) rule work? It’s not magic and now we’ll discuss the connection between the rule and its physical meaning. To understand the connection, we need to start with how the quantum numbers n and l are related to the energy of an orbital. We’ll use 3D models (actually 2D images of the 3D models) of atomic orbitals to demonstrate. [Sorry to disappoint those looking for a deep dive into quantum mechanical calculations. These models are visual representations of the results of those calculations.]
In Figure 3, we see a representation of the orbitals occupied by the electrons in the ground state of the element krypton (for clarity, the orbitals have been separated from one another). Notice that as the quantum number n increases (from 1 to 4 in krypton), so does the overall size of the orbital.
Figure 3. the electron configuration of krypton. (Generated using the Electron Configuration Lab of Atomsmith Classroom1)
How is the size of the orbital related to its energy? Recall that the potential energy of attraction between protons and electrons, which have opposite charges, depends on the distance between them: the closer an electron gets to the protons in the nucleus, the lower its energy will be. Compare the sizes of the 1s (n = 1) and 4s (n = 4) orbitals (Figure 3). Because the 1s orbital is smaller, the average distance of an electron to the nucleus will be smaller than that of the electrons in the 4s orbital. That’s the connection – the higher n is, the higher the energy of the orbital.
What about the l in the (n + l) rule? As mentioned above, l, the angular momentum quantum number, determines the shape of an orbital. In all orbitals for which n > 1, there are areas, called nodes, in which it is extremely unlikely to find an electron. There are two types of nodes: radial and planar (or angular). Figure 4 illustrates the radial node in a 2s orbital (l = 0) and a planar node in a 2p orbital (l = 1). Note that radial node (Figure 4, center) does not cross the nucleus, whereas planar nodes (Figure 4, right) do. s orbitals (which all have l = 0) contain only radial nodes. All other orbitals (p, d, f, etc., for which l > 0) contain both radial and planar nodes.
Figure 4. Left: 2s and 2p orbitals, overlapped. Center: radial node (l = 0) in a 2s orbital (green circle). Right: a planar node (l = 1) in a 2p orbital (green line). The 2s and 2p orbitals (center and right) have been “sliced” in Atomsmith’s Orbital Lab.
The total number of nodes (radial + planar) in an orbital is equal to (n – 1). Of these, l nodes are planar.
How does the number of planar nodes affect the energy of an orbital? Look again at the radial and planar nodes in Figure 4: the planar node crosses the nucleus – where the positively charged protons are. Radial nodes do not cross the nucleus.
If a node is an area where an electron is not likely to be found, then electrons in orbitals with planar nodes are likely to be found farther from the nucleus (on average). As we discussed earlier, large distances from the nucleus means higher energy. Thus, the higher the value of l, the more planar nodes an orbital has, and the higher the orbital energy.
So the (n + l) rule is a way to account for the two main factors that affect the relative energies of atomic orbitals: the size of the orbital (depends on n) and the number of planar nodes (= l). In cases where (n + l) is the same for two orbitals (e.g., 2p and 3s), the (n + l) rule says that the orbital with lower n has lower energy. In other words, the size of the orbital has a larger effect on orbital energy than the number of planar nodes.
Like all Models, Push Aufbau (n + l) Far Enough and it Fails.
The (n + l) rule is a model. And, as we tell our students, all models have limits. The (n + l) rule works quite well up to Z = 20, calcium (Z is the atomic number). What does “works well” mean? It successfully predicts two things:
- the relative energies of the orbitals
- the order in which the orbitals are occupied
It may not be obvious that these two things are different. But they are, and the differences start to matter at Z = 21, scandium – the beginning of the transition metals.
For Z = 20, calcium, the (n + l) rule says:
- the 4s orbital is lower energy than the 3d orbital
- the 4s orbital is occupied and the 3d orbitals are not (1s2 2s2 2p6 3s2 3p6 4s2).
These are both correct!
For Z = 21, scandium, the (n + l) rule says:
- the 4s orbital is lower energy than the 3d orbital
- the 4s orbital is occupied and one 3d orbitals is occupied (1s2 2s2 2p6 3s2 3p6 4s2 3d1).
Here’s where the (n + l) rule first fails. #2 (the occupation) is correct, but #1 is incorrect. For transitions metals, 3d is lower in energy than 4s! Figure 5 shows the relationship between orbital energy and atomic number (Z). Notice that the curves of the 4s and 3d orbital energies cross at Z = 21.
Figure 5. The relationship between orbital electronic energy and atomic number (Z). Vanquickenborne L.G., Pierloot K., and Devoghel, D. Transition Metals and the Aufbau Principle. J. Chem. Ed., 1994, 71(6) 469- 471.
However, and this is an important point: even though the (n + l) rule gets the orbital energies wrong, it still gets the electron configuration (orbital occupancies) right!
How is it possible that, for transition metals, the 3d orbitals are lower in energy, but they are not preferentially occupied?
The short answer is that the orbital energies are not the only important factor in determining how the orbitals are occupied. The long answer? It’s complicated – very complicated.
Prof. Dr. W.H. Eugen Schwarz, a theoretical chemist at the University of Siegen in Germany has published a number of papers on this very subject. His results are clearly beyond the scope of any introductory chemistry course, but we hope to give you a flavor of how other factors besides orbital energies may influence the occupancy of atomic orbitals in an electron configuration.
Schwarz elucidates five factors that influence the electron configuration of transition metals:2
- d-orbital collapse
- d versus s electron repulsions
- s Rydberg destabilization
- configurations and states in free and bound atoms
- relativistic spin-orbit coupling
We’ll look exclusively at the second factor: d versus s electron repulsions.
Let’s consider titanium (Z = 22). Its electron configuration is 1s2 2s2 2p6 3s2 3p6 4s2 3d2, which the (n + l) rule correctly predicts. If the electron configuration depended solely on the orbital energies, we would expect: 1s2 2s2 2p6 3s2 3p6 3d4 – with no electrons in the 4s orbital.
Why don’t the last four electrons preferentially occupy the 3d orbitals, which are lower in energy than the 4s orbitals?
Consider Figure 6a, where we see models of the 4s and 3d orbitals, separated in Atomsmith Classroom’s Electron Configuration Lab. If the electron configuration of titanium was 1s2 2s2 2p6 3s2 3p6 3d4, four of the five 3d orbitals would contain one electron each. And the 4s orbital would be unoccupied.
In Figure 6b, the 3d and 4s orbitals have been superimposed on one another around the nucleus. Just as protons and electrons attract each other due to their opposite charges, electrons repel each other because they have the same charge. This repulsion results in a higher energy – things are simply getting crowded.
What can the electrons do to minimize these repulsions? Notice that the 4s orbital is larger than the 3d orbitals. If two of these electrons find their way into the 4s orbital instead of 3d orbitals, they have more space to spread out to minimize the repulsion. This is the basis of Schwarz’s “d versus s electron repulsions.”
Figure 6a. The 4s and 3d orbitals separated.
Figure 6b. 4s and 3d orbitals overlapped.
How Can You Use This?
Let’s summarize what we’ve discussed so far:
Up to Z = 20 (calcium), the (n + l) rule (and the Aufbau diagram) correctly predicts:
- Orbital energy levels
The order of occupancy of the orbitals
- The physical meaning of the (n + l) rule (and its ability to make these predictions) is related to the size (n) and shape (l) of a given orbital.
For Z > 20 (starting at the transition metals):
- The (n + l) rule is not able to correctly predict orbital energy levels.
- Even when we know the orbital energies, this knowledge is not sufficient to predict the order of filling. Other factors, such as “d vs. s electron repulsions” (crowding) must be considered. (Schwarz discusses four more of these).
- Although its physical meaning is no long sufficient, the (n + l) rule still correctly predicts the order of filling. Except where it doesn’t and we invoke “exceptions.”
The first point to be taken from this is that the (n + l) rule is a model and that it works… until it doesn’t. If you choose to teach it as a model and connect it to some of the physical meaning discussed above, it’s a great example of how models can be both useful and also fail.
The “story” outlined above has the potential to be much more fulfilling for your students than “Memorize the diagram, learn to use it, and you’re guaranteed to get the right answer.” But it’s a tough story to tell by just waving your hands. You need a model to tell it, and the model needs the following features:
- Students must understand the basics of coulomb interactions: opposite charges are lower in energy when they are close together; the repulsions of like charges result in increased energy.
- Representations of the atomic orbitals that are physically accurate in both size (n) and shape (l)
- 3D is better than 2D
- The orbitals should be separable and superimposable
- Interactivity is desirable
You can’t tell this story without the ability to show your students the relative sizes and shapes (i.e., the nodes) of the orbitals (#2). Pictures of the orbitals in a textbook can work for many students; but all students will benefit from the ability to interactively observe (#5) the sizes and shapes in 3D (#3), and to separate and superimpose them (#4; Figures 6a and 6b) so that they can gain an appreciation for how crowded an atom really is.
The Aufbau principal, first envisioned by Niels Bohr in 1920, and its implementation as the (n + l) rule is a very useful abstraction. Connected to its physical meaning, it can become part of powerful mental model that students can draw on to build (and explain) their understanding of the structure of the atom. This kind of connection demonstrates the real promise of 3D particulate representations of atomic and molecular structure and phenomena. Many more of these kinds of stories will be told.
Author's note: The idea for this article arose from a discussion between the author and Tom Kuntzleman. Tom describes this interaction in a blog post, Conversations, Confessions, Confusions (and hopefully some Clarity) on Electronic Configurations.
References:
- Atomsmith Classroom, Bitwixt Software Systems, www.bitwixt.com(link is external). Available for Mac and Windows computers, and as an online HTML5 app for browsers on all platforms.
- Schwarz, W. H. E. The Full Story of the Electron Configurations of the Transition Elements, J. Chem. Educ., 2010, 87, 444 – 448.







All comments must abide by the ChemEd X Comment Policy, are subject to review, and may be edited. Please allow one business day for your comment to be posted, if it is accepted.
Comments 2
On electron configurations, Aufbau principle and its mnemonic
Hi:
Great article and very useful. I just want to comment on the Aufbau principle (n + l), and associated mnemonic shown above.
As with Madelung's Rule, these teaching devices cannot be arbitrarily applied across the entire Periodic System of Elements (PSE). The author is right in underscoring that models can fail.
I will add that it is misleading to consider the electron configurations of the ground states of free neutral atoms as the dominant configurations of bonded atoms in chemical substances.
Few years ago, I wrote a tutorial on this with the kind help of W.H.E. Schwarz, where a novel mnemonic describing the filling order of atomic orbitals according to the Rydberg Rule was presented. It is available online at http://www.minerazzi.com/tutorials/rydberg-rule-mnemonic.pdf. An electron configuration tool based on the tutorial was also developed.
The tutorial addresses the conceptual differences between Madelung and Rydberg rules, accounts for the predicted reordering of atomic orbitals and the large orbital energy gaps responsible for the periodicity of elements.
The proposed mnemonic illustrates that f orbitals are deeply buried beneath the valence shell, rarely playing a role in chemical change or bonding. This chemical fact can hardly be grasped from the traditional Madelung's mnemonic or Aufbau Principle that is blindfully taught at chemistry courses, and that cause a lot of confusion among students and even some teachers when they need to deal with transition element configurations.
Electronic Configuration
Hi
I understand the explanation. Now, it is so important to conclude in writing the correct electronic configuration of the transition element. I consider the correct one for Sc is [Ar] 3d1 4s2, while [Ar] 4s2 3d1 is definitely wrong, etc for the rest. What do you think?