
In a previous blog post I described some problems I encountered when beginning my instruction on energy this year. From the misconceptions fostered by the biology textbooks using the phrase “high-energy phosphate bond” to idea that energy comes in different forms, the Modeling community recognizes the challenges of teaching the energy concept and has developed a way of talking about energy designed to help students construct a consistent and cohesive model.
Three principles guide the model construction for energy:
- Energy is not a substance, but it is substance-like and can be stored in physical system.
- Energy can be transferred into and out of a system and can cause change.
- Energy does not change its identity after being transferred.
We use metaphors to help clarify what is meant by “substance-like”. We examine how we talk about information. Information is not a substance, just as energy is not. However, information can behave like a substance. Information can be stored in books, on CD’s, on computers – it can also be transferred from one place to another via Bluetooth, the Internet, or through cables. Most importantly, for the purpose of our discussion, when information is transferred from one place to another the information itself does not change. This is an important parallel to draw between energy and the information metaphor. When energy is transferred from one place to another, it remains energy. Nothing about the energy itself has changed.
The second metaphor we present is the money metaphor. We talk about different ways we can store money. Students provide examples of different types of accounts at banks. We talk about how each type of account – savings, checking, money market, etc. – stores one thing. They store money. Is $100.00 in a savings account worth a different amount if it is transferred into a checking account? No – money can be moved between accounts and it still retains its original identity, its value remains the same. The same is true for energy.
The Modeling Curriculum uses the concept of accounts discussed in the money metaphor to begin to build the model of energy storage and transfer used in both the Physics and Chemistry Modeling curriculums. We establish different types of “accounts’” to help students keep track of energy as it is transferred. The discussion of energy accounts immediately following the examination of the money metaphor helps students start to see that the act of transferring energy does not change the identity of the energy. The Chemistry curriculum is concerned with three accounts:
The Thermal Energy (Eth) Account
Energy in this account is the energy stored by the movement of particles. The quantity of energy is related to the mass and velocity of the particles in the system. This energy can be described by the temperature of the system. Warmer temperatures equate to more energy in the thermal account.
The Phase Energy (Eph) Account
Energy in this account is the energy stored in the system due to the arrangement of the particles and the interactions between those particles. Greater attraction decreases the energy of the system. In other words, as the particles are more tightly bound – more attracted to one another – the energy of the system decreases. Solids possess the least amount of energy in this account, followed by liquids. Gases have the most.
The Chemical Potential Energy (Ech) Account
Energy in this account is the energy due to attractions within molecules.
Energy Transfer
Once we have built the model for energy storage we introduce the methods of energy transfer. Traditional texts will name these methods work, heat, and radiation. We will refer to them as working (W), heating (Q), and radiating (R). While this difference may seem subtle, it is actually a very powerful and purposeful change. Using the terms in gerund form emphasizes they these are actions – they are processes and not things separate from energy. During the discussion of energy transfer we highlight the fact that when energy is transferred it affects both the system and surrounds. Energy doesn’t just appear or disappear. It comes from somewhere and when it leaves, it goes somewhere.
Working
This is the process where energy is transferred between macroscopic objects that exert forces on one another. This method is discussed more often in the physics curriculum.
Heating
This is the process by which energy is transferred through collisions of microscopic objects. In this process energy is always transferred from the hotter object to the cooler. This is the process we use most often in the Chemistry curriculum.
Radiating
This is the process where energy is transferred by releasing or absorbing photons.
The modeling curriculum then synthesizes these ideas into a tool we use to illustrate energy changes in both physical and chemical processes.
The Energy Bar Charts

My students call these LOL charts. Can you see why?
The energy bar chart is the tool we use to help students describe what is happening to energy in a system under different conditions.
Lets consider an example:
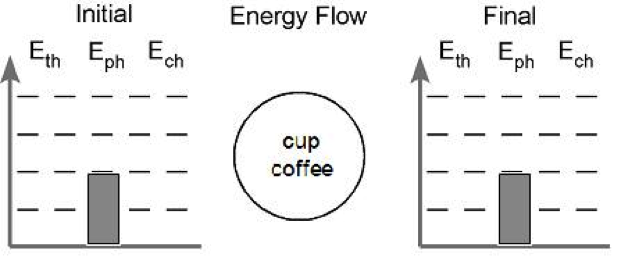
Conditions: A cup of hot coffee is allowed to cool on a table.
First, we establish what the system is. The system in this case is the cup of coffee. We would write the phrase “cup of coffee” in the circle at the center of the chart. The circle represents our system.
Second, we determine if a chemical change will occur. We start with coffee and end with coffee so no chemical change takes place. We can ignore the Ech account for now.
Next, we assign values to the energy in the phase account. We know that solids have the least amount of energy here due to the strong interactions between the particles. Liquids have slightly more energy than solids and gases have a lot more energy then either other phase. We can establish a convention to help discussion run more smoothly. Solids can be assigned one bar of energy and liquids will be given two. To illustrate gases having significantly more energy we can assign them four bars. In our problem, we start and end with coffee, which is a liquid. Applying these conventions, our diagram now looks like this:
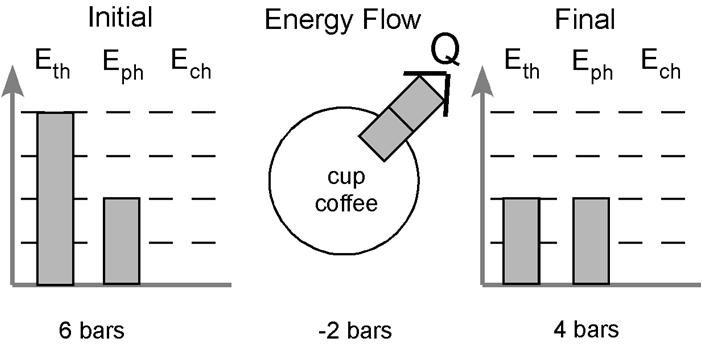
Lastly, we look at energy stored in the thermal account. The coffee begins hot and cools down. We need to show this in our picture. We can begin by assigning four bars of energy to the thermal account when the coffee is hot and showing a decrease in the amount of energy in the final picture. I usually use two bars to represent room temperature, so lets assign two bars to the thermal account in the final picture. The picture now looks like this:
Okay, so we begin with a total of six bars in the initial picture but end with only four bars at the final. Where did those two bars go? They were released to the surroundings. What method of transfer is used? Well, the only change taking place is the cooling of the system. The particles are slowing down because some of the energy is being transferred to the surroundings. This is energy transfer via heating. We can label the bars leaving with the letter “Q” to indicate we know the energy is leaving via heating. We can illustrate this by drawing the two bars leaving the system – leaving the circle in the center.
By looking at the diagram I can glean a lot of information about what my students think is going on in this situation. I see they know there is no chemical change in this example. I can also immediately see they understood no phase change took place and that the process is exothermic. The diagram also indicates the system starts hot and cools down. All of this information is conveyed without writing a single word.
Here is a video of one of my students sharing her ideas on the same example:
This is just one example of how energy bar charts can be used to illustrate energy changes. Consider the example which includes a phase change:
Conditions: A tray of ice cubes at -8.0oC is left on the counter. It melts and warms to 20.0oC. What would your EBC look like for this situation?
(I’ll post the solution in the comments)
The role of energy in chemistry is a complex and difficult topic to teach. This is just a small sampling of how the Chemistry Modeling Curriculum addresses some of the difficulties chemistry teachers face when trying to teach this abstract concept. My next post will look at how EBCs are used when chemical change occurs.
NGSS
Students who demonstrate understanding can use a model to describe how variations in the flow of energy into and out of Earth’s systems result in changes in climate.
More information about all DCI for HS-ESS2 can be found https://www.nextgenscience.org/dci-arrangement/hs-ess2-earths-systems and further resources at https://www.nextgenscience.org.
Students who demonstrate understanding can use a model to describe how variations in the flow of energy into and out of Earth’s systems result in changes in climate.
Assessment of the results of changes in climate is limited to changes in surface temperatures, precipitation patterns, glacial ice volumes, sea levels, and biosphere distribution.
Examples of the causes of climate change differ by timescale, over 1-10 years: large volcanic eruption, ocean circulation; 10-100s of years: changes in human activity, ocean circulation, solar output; 10-100s of thousands of years: changes to Earth's orbit and the orientation of its axis; and 10-100s of millions of years: long-term changes in atmospheric composition.
Students who demonstrate understanding can develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.
*More information about all DCI for HS-PS1 can be found at https://www.nextgenscience.org/dci-arrangement/hs-ps1-matter-and-its-interactions and further resources at https://www.nextgenscience.org.
Students who demonstrate understanding can develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.
Assessment does not include calculating the total bond energy changes during a chemical reaction from the bond energies of reactants and products.
Emphasis is on the idea that a chemical reaction is a system that affects the energy change. Examples of models could include molecular-level drawings and diagrams of reactions, graphs showing the relative energies of reactants and products, and representations showing energy is conserved.


All comments must abide by the ChemEd X Comment Policy, are subject to review, and may be edited. Please allow one business day for your comment to be posted, if it is accepted.
Comments 9
Answer to questions posted above
ice.png
Question
Love the idea. I am wondering about the potential energy graphs that I am used to drawing with my students though. Do you still end up using them for explaining endothermic and exothermic? Do you use them to discuss activation energy / catalysts or do you just use these graphs?
Potential Energy Diagrams
Deanna,
Good Question! I actually use them when I talk about chemcial changes, because I know my students could potentially run across them on a standardized test at some point. I do not use them to teach the topic of endo/exothermic. I use the EBC's to discuss what is meant by each type and what happens to the energy entering or exiting the system. I show students the potential engeryg diagrams after they can already explain endo/exothermic. I use it as an example of an alternative way to represent the same ideas. Some modelers abandon those completely simply because they do not tell the whole story of energy changes.
I think the model
has severe flaws by calling energy substance like. In fact I think that it is a huge mistake to do anything but break down why energy is fictitious and not real. We should be trying to better explain chemical phenomena in terms of position and its derivatives and force. Energy is a great math shortcut, especially for chemistry with so many particles that using statistics instead of reality makes perfect sense. But I feel like this model is rife with misconception and too much shortcut. Perhaps modelling is not appropriate for energy or maybe there is one that works well, but this is going to be a disaster for understanding.
I respectfully disagree
I respectfully disagree with the argument made by Scott Milam in his response to Erica Adam-Posthuma's post about energy. I believe that metaphors are very powerful tools for helping us make sense of the world. Consider for a moment how we try to make sense of "light". We call it a particle or a wave when it is actually neither. Nonetheless, these metaphors are useful for helping understand and predict its behavior in different situations. The same reasoning applies to energy. While we no longer believe that energy is "substantial" (like caloric), the use of the substance metaphor helps us understand important aspects of energy.
These are all properties/behaviors of a substance; hence, we describe energy as substance-like.
Since I have adopted the treatment of energy promoted by Modeling Instruction I have found that my students (and the teachers I have worked with) have felt much more comfortable with the role energy plays in change. They have a set of tools (which Erica has outlined nicely) for describing ways that a system can store energy and how energy can be transferred between system and surroundings. This deep conceptual understanding also helps them with their quantitative treatment of energy. I think the use of this metaphor is much more satisfying than the approach that energy is simply "invented" to help us with calculations.
How does your model address these questions?
If one tries to answer the following, the shortcomings of your model may become apparent.
Until these questions can be answered, then the kritik would still stand. The issue is not understanding energy for a physics problem; it's the vast gap of understanding that occurs because of the limits of not addressing the reality of energy as being a mathematical shortcut. The model given will make this problem worse and undermine your teaching. Look at the answers students make to some state testing problems about energy. You will see the fact that students cannot answer correctly because they have a very limited understanding (even a misconception) of force/motion because of energy especially as taught in chemistry. This may might be the biggest problem in science education. Do not dismiss this issue and reconsider the approach.
Curious
I am in the middle of a Modeling Workshop learning to use the energy diagrams that Erica describes. I have been thinking that it the presentation will be more intuitive than the way I have taught about energy in the past. I know that we will be using these models and modifying them as we progress through the coursework and students discover more things about particles. I am used to using models and discussing misconceptions. Every model will have some misconceptions. I am not clear about your suggestion. What/how should we teach instead of using these diagrams? I am sincerely interested in understanding what you are suggesting. Deanna
a tough question!
Here's a youtube series I'm starting this summer that will explain things without using energy when I can. Around 8 or 9 minutes I go through endothermic and exothermic without using energy https://www.youtube.com/watch?v=ALLrFIkurgY
I would avoid modeling for energy personally. When I teach I mostly just lecture and have students work in groups on things, so I wouldn't be the person to look for a fancy teaching methodology from. But when teaching thermochemistry you should point out that it is a huge shortcut and emphasize how convenient it is to use energy to calculate in place of looking at individual motion and forces for molecules. That would be impossible with the sheer numbers and inability to gather data. And the shortcut will produce an answer and it will be correct or close enough to correct, but that it's not a thing. It's a human or mathematical or scientist contrived shortcut to solve problems. It should never be an explanation as to why something happens. We should avoid asking questions that ask why does this happen with the expectation of an answer that says because it has a lot of energy or because it's more stable. All of these questions we ask can be explained using forces, positions and derivatives of position (velocity, speed, acceleration). A good example is bond formation. We say that bond formation is exothermic and students don't believe. They'll write it for an exam, but they don't actually believe it and it's very tough to break this misconception down. But if instead of saying that forming a bond releases energy, we said that when you start with these two atoms far apart, and as they move closer together they speed up, kids would understand that. They've experienced that and it's intuitive to them because it's similar to gravitational effects. Now the atoms are going to be moving faster, they will accelerate towards each other. Once they've bonded and are now moving in some direction at a high speed, they can collide at a high speed with something else causing another bond to be separated by the force of repulsion during the collision. This will slow down the parts of the molecules that have the bond breaking because the force of that bond is pulling on them with a force that opposes the direction of the motion prior to the collision.
Some of that might be beyond the level you're teaching, which is totally fine, but we shouldn't present energy in a confident manner to build a false sense of confidence in it. It undermines the future classes and the ability of the students to learn more in the future, which I think if you look you'll see the very few people can explain chemistry without energy.
Exothermic is so difficult?
Using the energy bar chart can help quite a lot understanding that total chemical energy of products is lower than the total energy of reactants, so that if you consider energy conservation, in order to have energy conservation, you have to consider a type of energy which is not chemical and it is thermal one. If the reaction is endothermic, it is probably because the total chemical energy of reactants is lower than the total energy of products and you have to consider unstable intermediary states of higher energy in order to obtain the final product. Of course things are not so simple but discussing energy is, I think, a pretty good start. When we think of two atoms forming a molecule, it is quite easy to understand that the reaction is exothermic, the kinetic energy is increased. If not, the atoms may not form a molecule but part away. The dynamic picture is interesting, useful but, I think, we cannot avoid energy discussions.