
As I planned for my mole unit this year, I thought about how fundamental the mole concept is for stoichiometry and brainstormed ways to really make it stick. I usually do a lab practicum at the end of every unit. I have shared about my Lab Practicums in Chemistry before. For the mole unit, I decided to do multiple mini-practicums, one for each learning target of the unit. Here are my learning targets and a brief description of the mini-practicums I did.
Target 1: I can convert between mass and moles of an element or compound.
For this learning target, I gave students the “mole mug brownie” recipe and conversion chart (figure 1) and asked them to convert the measurements to the dreaded imperial units we use to bake with.

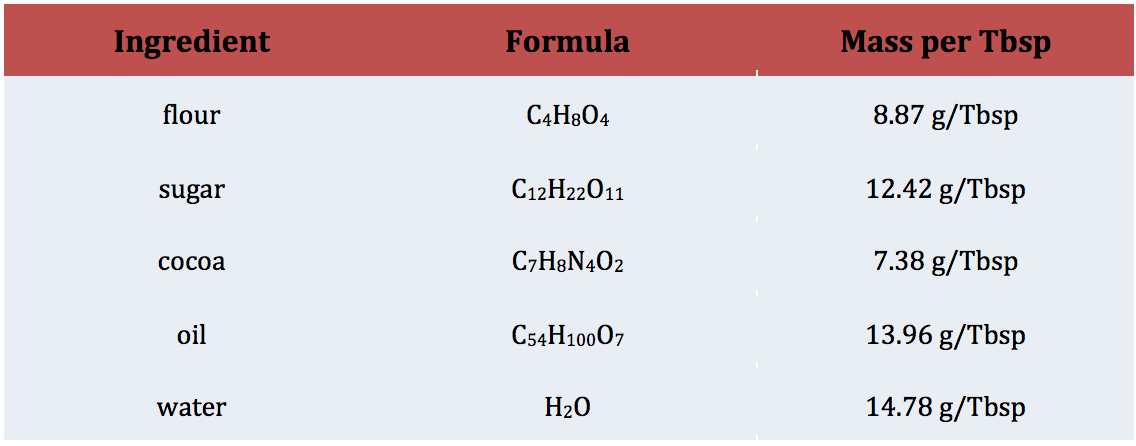
Figure 1: Recipe and Data for Converting Units
After students did all of their math, they did get to test out their calculations by actually making the mole mug brownie. Reviews on the actual brownie were mixed but the math concepts definitely stuck!
Target 2: I can convert between the number of particles and moles of an element or compound.
For this learning target, I gave each group a stick of chalk and the challenge in figure 2.

Figure 2: Directions for Chalk Challenge
While this practicum did not have an edible element, students really enjoyed writing on their lab stations (see figure 3) with chalk!

Figure 3: Chalk Signature on Lab Counter
Target 3: I can relate the molar concentration (molarity) of a solution to the number of moles and volume of the solution.
For this learning target, I tried something I had never done before. I gave each group a small (50 - 100mL) volumetric flask and a given molarity (different molarity for each group). It was up to each group to mix a solution of copper (II) chloride to their given molarity. I tested each group’s solution using my Vernier SpectroVis and a Beer’s Law curve I had created the day before. Students thought the spectrometer was pretty cool and enjoyed the instant confirmation that they mixed their solution correctly.
Target 4: I can determine the empirical formula of a compound given the mass or percent composition.
For this learning target, I used my typical end of the unit practicum, find the formula of a hydrate. I have used different hydrates in the past but this year I used copper (II) sulfate pentahydrate. If you have not done this lab before, it is super simple and yields reliable data. Students heat their hydrate in a crucible to drive off all of the water. They use the before and after masses to calculate their moles of water in their sample and ratio it to their moles of copper (II) sulfate. The only thing I provide my students before they begin is a brief introduction to hydrates and an overview of how to heat a sample using a crucible.
I also have a learning target for molecular formulas (I can determine the molecular formula of a compound given the mass or percent composition and molar mass) but I did not make a separate practicum for it. Instead, I gave students the accepted molar mass for the copper (II) sulfate pentahydrate they were testing to compare their lab data to.
Since the mole unit involves a lot of practice, these practicums were a nice way to break up the worksheet/whiteboarding cycle, keep students engaged and reinforce concepts!
Safety
General Safety
General Safety
For Laboratory Work: Please refer to the ACS Guidelines for Chemical Laboratory Safety in Secondary Schools (2016).
For Demonstrations: Please refer to the ACS Division of Chemical Education Safety Guidelines for Chemical Demonstrations.
Other Safety resources
RAMP: Recognize hazards; Assess the risks of hazards; Minimize the risks of hazards; Prepare for emergencies
NGSS
Analyzing data in 9–12 builds on K–8 and progresses to introducing more detailed statistical analysis, the comparison of data sets for consistency, and the use of models to generate and analyze data.
Analyzing data in 9–12 builds on K–8 and progresses to introducing more detailed statistical analysis, the comparison of data sets for consistency, and the use of models to generate and analyze data. Analyze data using tools, technologies, and/or models (e.g., computational, mathematical) in order to make valid and reliable scientific claims or determine an optimal design solution.
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories.
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories. Construct and revise an explanation based on valid and reliable evidence obtained from a variety of sources (including students’ own investigations, models, theories, simulations, peer review) and the assumption that theories and laws that describe the natural world operate today as they did in the past and will continue to do so in the future.
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories.
Constructing explanations and designing solutions in 9–12 builds on K–8 experiences and progresses to explanations and designs that are supported by multiple and independent student-generated sources of evidence consistent with scientific ideas, principles, and theories. Construct and revise an explanation based on valid and reliable evidence obtained from a variety of sources (including students’ own investigations, models, theories, simulations, peer review) and the assumption that theories and laws that describe the natural world operate today as they did in the past and will continue to do so in the future.
Planning and carrying out investigations in 9-12 builds on K-8 experiences and progresses to include investigations that provide evidence for and test conceptual, mathematical, physical, and empirical models.
Planning and carrying out investigations in 9-12 builds on K-8 experiences and progresses to include investigations that provide evidence for and test conceptual, mathematical, physical, and empirical models. Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data (e.g., number of trials, cost, risk, time), and refine the design accordingly.
Mathematical and computational thinking at the 9–12 level builds on K–8 and progresses to using algebraic thinking and analysis, a range of linear and nonlinear functions including trigonometric functions, exponentials and logarithms, and computational tools for statistical analysis to analyze, represent, and model data. Simple computational simulations are created and used based on mathematical models of basic assumptions. Use mathematical representations of phenomena to support claims.
Mathematical and computational thinking at the 9–12 level builds on K–8 and progresses to using algebraic thinking and analysis, a range of linear and nonlinear functions including trigonometric functions, exponentials and logarithms, and computational tools for statistical analysis to analyze, represent, and model data. Simple computational simulations are created and used based on mathematical models of basic assumptions. Use mathematical representations of phenomena to support claims.
Students who demonstrate understanding can use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction.
*More information about all DCI for HS-PS1 can be found at https://www.nextgenscience.org/dci-arrangement/hs-ps1-matter-and-its-interactions and further resources at https://www.nextgenscience.org.
Students who demonstrate understanding can use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction.
Assessment does not include complex chemical reactions.
Emphasis is on using mathematical ideas to communicate the proportional relationships between masses of atoms in the reactants and the products, and the translation of these relationships to the macroscopic scale using the mole as the conversion from the atomic to the macroscopic scale. Emphasis is on assessing students’ use of mathematical thinking and not on memorization and rote application of problem - solving techniques.

All comments must abide by the ChemEd X Comment Policy, are subject to review, and may be edited. Please allow one business day for your comment to be posted, if it is accepted.
Comments 8
Thank you!
Lauren, I love your ideas. Just wanted to say thanks. I listened to your webinar about Standards based grading on AACT. I plan to start next year. Thanks for all that you do!
Thanks for the encouraging
Thanks for the encouraging words Chad! I'm excited about your SBG journey! Email me if you ever have any questions!
Brownies...
Do they mix their ingredients together in a coffee cup and then microwave it? How long? I like this idea :)
I had them do 1 full recipe
I had them do 1 full recipe/group and then divide the batter into Dixie cups. It took about 45 seconds in my low power microwave but you have to play around with it.
Hi Lauren, I love these ideas
Hi Lauren, I love these ideas and will actually be doing a variation when we get to stoichiometry. A recipe is essentially a balanced chemical equation so they will be given a recipe for a cake, that does not require eggs, and asked to reduce it to 1/3 and then mix and bake their cake. I shall definitely refer to your ideas when I'm doing my edits! Thanks for the inspiration.
That sounds like fun! I do
That sounds like fun! I do the s'more stoichiometry activity going into stoich which sounds similar and always goes well!
Brownies...one more time
What kind of oil do you use?
I used vegetable oil though I
I used vegetable oil though I'm sure it would work fine with other oils.