
I enjoyed reading Yvonne Clifford’s piece, The Diet Coke And Mentos Reaction – Having A Literal Blast!, regarding the sublimation of dry ice, CO2(s). The activities she describes are original and highly engaging, with considerable “WOW” factor.
My use of dry ice in a demonstration—not a student activity—is less visually appealing, but still visually appealing, if you catch my drift.

Figure 1. Dry ice, CO2(s), added to water. The CO2 sublimates; the “steam” is condensed water vapor.1
I use dry ice to illustrate an application of phase diagrams. I start by allowing students to observe the sublimation of dry ice; I add some to a beaker of water for dramatic effect, as shown in figure 1.
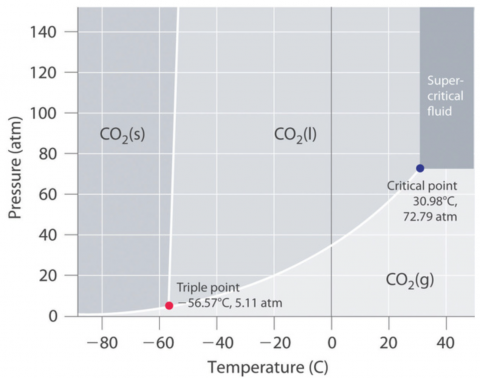
Figure 2. Phase diagram for carbon dioxide, CO2. Reused from Libretexts Phase Diagrams.2
As you can see from the Phase Diagram for CO2 (Figure 2), sublimation occurs at standard pressure (1 atm, 101 kPa) and temperature (25°C). This physical property is illustrated in Yvonne’s activities.
Notice that at pressures above 5.11 atm, dry ice will melt at room temperature.
I demonstrate this by placing dry ice into an empty, clear 2-L soda bottle and tightening the cap. A cursory look online confirms that 2-L soda bottles can withstand something like 10 atm of pressure.3 As the CO2 sublimates, the pressure inside the bottle increases. When it exceeds 5.11 atm, the CO2 liquefies.
Equipped with this knowledge, and using the Ideal gas Equation4, I add about 25 g of dry ice to an empty 2-L soda bottle and quickly tighten the cap. Without delay I place the bottle behind a safety shield, situated in the fume hood—and lower the plexiglass shield of the fume hood. This gives two layers of protection should the bottle explode—which it never has.
In a short while, clear, colorless liquid CO2 appears, which induces geekish amazement, even on a Friday afternoon.
Next, I ask students to predict what they expect to see when the cap is unscrewed, based on the phase diagram of CO2. Then, donning ear protection, a face shield, and protective gloves5, I reach behind the safety shield to unscrew the cap.
We are instantly rewarded with a “whoosh” as CO2(l) instantly vaporizes as the pressure is reduced to 1 atm.
Safety Note:6 Set up the demonstration as described. Use a new soda bottle free of cracks, nicks and weak spots. Handle dry ice carefully; wear gloves; dry ice is super-cold (–78.5°C ; –109°F). Wear ear protection in case the bottle explodes. Initially, perform the demonstration without students present.
Dry ice is commercially available; one kg should last through a bunch of classes, as long as it’s kept in a non-airtight cooler and used on the same day.
Together with Yvonne’s activities, this demonstration gives a fulsome, Physical Chemistry-based look at dry ice.
References
-
Photo credit - Tom Kuntzleman
-
Reprinted from Libretexts Chemistry 12.4: Phase Diagrams, 2020. https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_General_Chemistry_(Petrucci_et_al.)/12%3A_Intermolecular_Forces%3A_Liquids_And_Solids/12.4%3A_Phase_Diagrams (accessed 2/21/2023).
-
Andrea Becker, How Much Pressure Can a Two Liter Bottle Handle?, Seattle pi, accessed 2/2/2023.
-
PV = nRT; 25 g CO2 = 0.57 mol, which results in a pressure of about 7 atm in the bottle
-
I wear light winter gloves that afford protection from the cold dry ice, while still allowing me to unscrew the bottle cap.
Safety
General Safety
General Safety
For Laboratory Work: Please refer to the ACS Guidelines for Chemical Laboratory Safety in Secondary Schools (2016).
For Demonstrations: Please refer to the ACS Division of Chemical Education Safety Guidelines for Chemical Demonstrations.
Other Safety resources
RAMP: Recognize hazards; Assess the risks of hazards; Minimize the risks of hazards; Prepare for emergencies
