
Stoichiometry is one of the most fundamental topics in a high school chemistry course, but it is also one of the most challenging concepts for students to master.1 Stoichiometry is abstract, making it challenging to learn and teach.2 As with any abstract concept, activities that encourage conceptual thinking tend to promote a deeper level of understanding.3
In this activity, a video introduces stoichiometry and guides students to think conceptually using a simple baking analogy. Afterward, stoichiometry calculations connect to the analogy, that are then reinforced with a simple experiment. Finally, students explore a PhET simulation to deepen their knowledge.
This activity can be completed in one 50 minute class period. The extension “at home” section would require about 50 more minutes.
Section 1 and 2 only require videos and the PhET simulation, "Reactant, Products and Leftovers", Section 3 (at-home investigation) requires 1 effervescent tablet, 35 mL of vinegar, a large cup, electronic balance, parchment paper, 100-mL graduated cylinder.
Section 1 - Introducing Stoichiometry
The activity begins with a short video (video 1) that introduces stoichiometry with a simple baking analogy. After answering related questions on a worksheet (see supporting information), the video teaches students to perform stoichiometric calculations and then applies them in a simple experiment.
Video 1: Chemistry Experiment: Does Alka-Seltzer Tell the Truth? on The Science Classroom YouTube Channel (Published 11/29/2020)
Section 2 - Conceptualizing Stoichiometry
In the PhET simulation, Reactants, Products and Leftovers (image 1), students think conceptually about stoichiometry with sandwich making and particle level simulations.
Image 1: "Reactants, Products, and Leftovers" PhET simulation (accessed 12/15/2020).
Section 3 - Try it at Home
The final, optional section of the activity provides instructions for an experiment to determine the amount of sodium bicarbonate in an Alka-Seltzer tablet.4 The procedure is simple enough that students can perform it in their kitchen at home.
References
- Kimberlin, S. & Yezierski, E. (2016). Effectiveness of Inquiry-Based Lessons Using Particulate Level Models to Develop High School Students’ Understanding of Conceptual Stoichiometry, Journal of Chemical Education. 93, 1002−1009
- BouJaoude, S. & Barakat, H. (2000). Secondary school students’ difficulties with stoichiometry, School Science Review, 81, 91-98.
- Chandrasegaran, A. L., Treagust, D. F., Waldrip, B. G. & Chandrasegaran, A. (2009). Students’ dilemmas in reaction Stoichiometry problem-solving: deducing the limiting reagent in chemical reactions, Chemistry Education Research and Practice, 10, 14-23.
- Chen, Y. H., & Yaung, J. F. (2002). Alka-Seltzer Fizzing-Determination of Percent by Mass of NaHCO3 in Alka-Seltzer Tablets. An Undergraduate General Chemistry Experiment, Journal of Chemical Education, 79(7), 848.
Students work through the Virtual Lab: Stoichiometry assignment document (found in the Supporting Information) as described below (image 2). Log into your ChemEd X account for access. The document includes all of the required links to videos and PhET simulations.
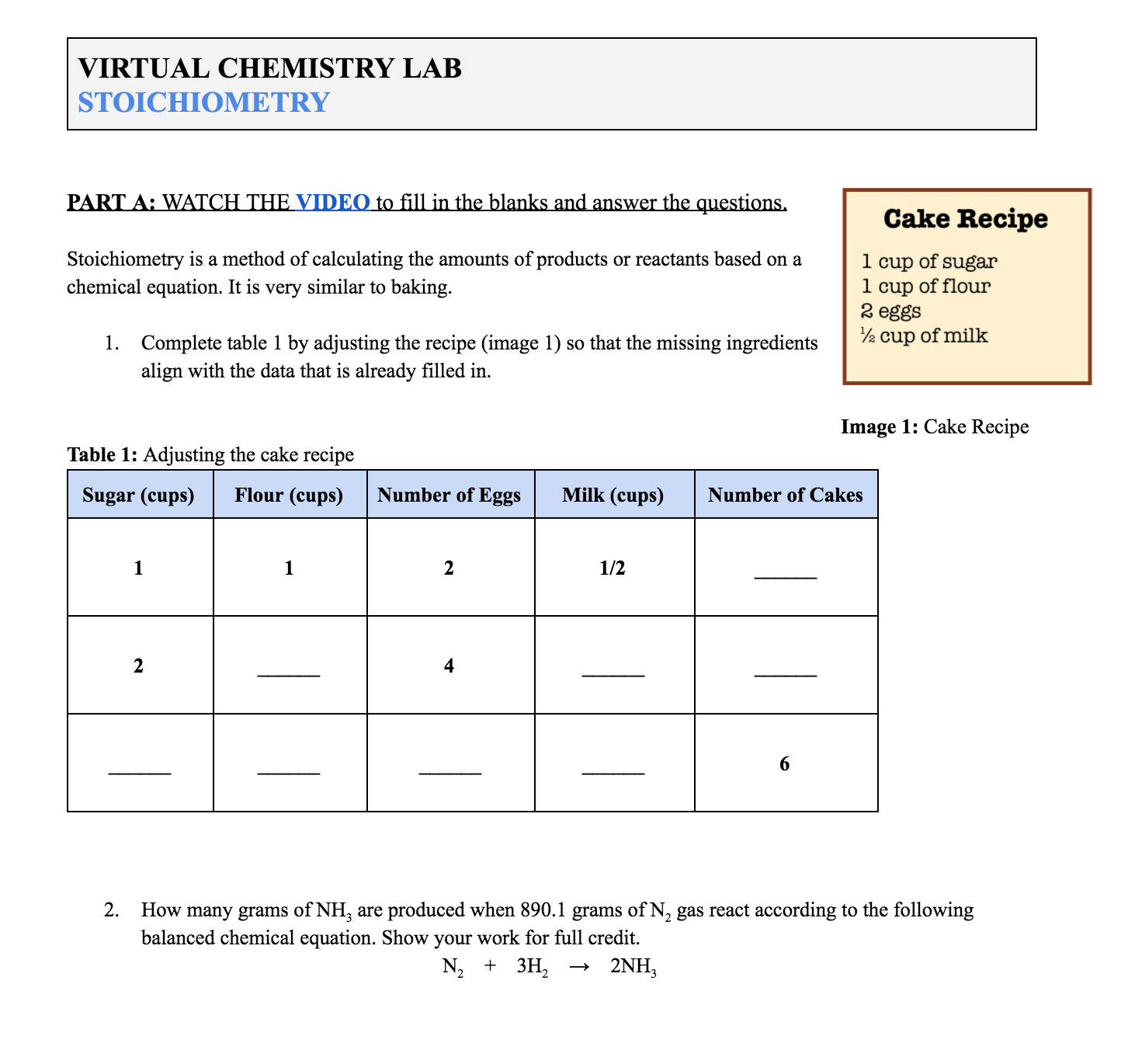
Image 2: Example of questions in the student document (available in the Supporting Information)
The student document, including questions, can be found in the Supporting Information below. Log into your ChemEd X account for access.
No teacher preparation is required unless completing the "at home" portion as a demonstration for students.
YouTube Channel: The Science Classroom
Simulation: PhET
Safety
General Safety
General Safety
For Laboratory Work: Please refer to the ACS Guidelines for Chemical Laboratory Safety in Secondary Schools (2016).
For Demonstrations: Please refer to the ACS Division of Chemical Education Safety Guidelines for Chemical Demonstrations.
Other Safety resources
RAMP: Recognize hazards; Assess the risks of hazards; Minimize the risks of hazards; Prepare for emergencies
NGSS
Mathematical and computational thinking at the 9–12 level builds on K–8 and progresses to using algebraic thinking and analysis, a range of linear and nonlinear functions including trigonometric functions, exponentials and logarithms, and computational tools for statistical analysis to analyze, represent, and model data. Simple computational simulations are created and used based on mathematical models of basic assumptions. Use mathematical representations of phenomena to support claims.
Mathematical and computational thinking at the 9–12 level builds on K–8 and progresses to using algebraic thinking and analysis, a range of linear and nonlinear functions including trigonometric functions, exponentials and logarithms, and computational tools for statistical analysis to analyze, represent, and model data. Simple computational simulations are created and used based on mathematical models of basic assumptions. Use mathematical representations of phenomena to support claims.
Students who demonstrate understanding can use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction.
*More information about all DCI for HS-PS1 can be found at https://www.nextgenscience.org/dci-arrangement/hs-ps1-matter-and-its-interactions and further resources at https://www.nextgenscience.org.
Students who demonstrate understanding can use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction.
Assessment does not include complex chemical reactions.
Emphasis is on using mathematical ideas to communicate the proportional relationships between masses of atoms in the reactants and the products, and the translation of these relationships to the macroscopic scale using the mole as the conversion from the atomic to the macroscopic scale. Emphasis is on assessing students’ use of mathematical thinking and not on memorization and rote application of problem - solving techniques.

