
In the January, 2018 issue of the Journal of Chemical Education, Jeffrey Statler describes several experiments that can be conducted with liquid air.1 He demonstrates that liquid air can be easily collected by simply immersing a test tube in liquid nitrogen, which has a temperature of 77 K. Upon doing so, the air within the test tube is cooled to below the condensation point of air (79 K). Thus, any air that enters the test tube condenses to a liquid. About 20 mL of liquid air can be collected if the test tube is left undisturbed in the liquid nitrogen for 30 minutes. If a strong neodymium magnet is immersed into the liquid air thusly collected, liquid oxygen (which is paramagnetic) is attracted to the magnet!
I have used the method described by Statler several times to condense liquid air. In fact, I have also used other methods to condense air from the atmosphere (scroll below to view a video that displays how to conduct another simple and useful method to condense air). However, I learned several important things by reading Statler’s article. For example, I learned that the liquid collected in this experiment is air and not pure oxygen. Thus, the liquid collected contains mostly a mixture of about 78% nitrogen and 21% oxygen. I also learned that a neodymium magnet can be used to separate some oxygen from the liquefied air. This is fascinating experiment to carry out if you get a chance to do so!
An additional method I have used to extract liquid air from the atmosphere has the advantage that it allows observers to directly see droplets of condensed air form and fall into a container for collection. To do this, a roughly-ridged, deep aluminum pan is filled ¼ full with liquid nitrogen. Next, a corner of the pan is suspended above a Styrofoam cup. The nitrogen cools the pan to a low enough temperature that liquid droplets immediately begin to fall from the corner of the pan and into the cup. Several milliliters of fluid can be collected within 5 – 10 minutes using this protocol. After reading Statler’s article, I decided to collect and test the liquid obtained using this secondary method. You can see the experimental set up and some of my tests in the video below:
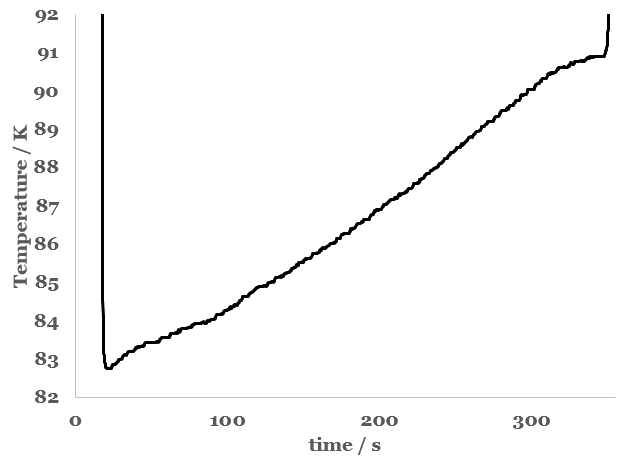
After several experiments, my hunch is that the liquid collected is oxygen-rich air. I think this based on measurements of the boiling temperature of the liquid collected (Figure 1). Note that the boiling point of the condensed liquid usually starts out around 83 K, and rises to slightly over 90 K before entirely boiling away. The boiling point of the mixture starts somewhat higher than the boiling point of air (79 K), but lower than the boiling point of oxygen (90 K). Over time, the boiling point of the condensed liquid rises, tending toward the boiling point of oxygen. Thus, it is likely that the air collected is enriched in oxygen because nitrogen gas preferentially boils away due to its greater volatility. For comparison (data not shown) the boiling point of a sample of liquid oxygen was found to remain steady at around 90 – 91 K for several minutes. Likewise, the boiling point of a sample of liquid nitrogen remained steady at around 76 – 77 K for several minutes.
Figure 1 - Boiling point of the liquid collected from an aluminum pan cooled with liquid nitrogen. The initial drop in temperature is due to initial cooling of the thermocouple after immersion in the very cold liquid. The final increase in temperature is due to warming of the thermocouple after the liquid has completely boiled away.
I would certainly enjoy hearing from you if you try out some of these experiments, have suggestions for further experiments, or otherwise have any comments. What are some simple ways you might use to collect liquid air or other liquefied gases?
Happy experimenting!
Reference:
1. Statler, Jeffrey, Microscale Extraction of Liquid Oxygen from a Cryogenic Mixture Formed through Condensation of Ambient Air, Journal of Chemical Education 2018 95 (1), 116-120. https://pubs.acs.org/doi/10.1021/acs.jchemed.7b00436

All comments must abide by the ChemEd X Comment Policy, are subject to review, and may be edited. Please allow one business day for your comment to be posted, if it is accepted.
Comments 2
Industrial Nitrogen
I enjoyed reading the experiments with liquid air. The process described (and the figure) represent the industrial process by which nitrogen and oxygen (and argon) are separated from air. The air is liquefied by compression/expansion cycles and then fractionally distilled with the nitrogen boiling off first. Thus the liquid remaining is enriched in oxygen. Liquid air presents a hazard because as the nitrogen boils off the liquid becomes liquid oxygen and is a very strong supporter of combustion. You can test for the presence of oxygen gas in the test tube after all of the liquid air boils off using a small wood splint that has been ignited an blown out, leaving a glowing ember. When dropped into the test tube (make sure all the liquid is goine) it will re-ignite--old fashioned test for oxygen gas. Don't put anything combustible into the tube while the liquid oxygen is still there because it can inflame very fast.
I appreciate your comments
I appreciate your comments and added insight, John. By the way, I will soon be trying your suggested experiment with the test tube and oxygen!